Tresiba® receives positive opinion from CHMP for treatment of children with diabetes
Copenhagen, Denmark, 19 December 2014 – Novo Nordisk today announced that the Committee for Medicinal Products for Human Use (CHMP) has issued a positive opinion for expanded use of Tresiba® (insulin degludec) in children and adolescents aged 1-17 years with diabetes. Once the European Commission approves the label expansion, physicians in the European Union will be able to prescribe Tresiba® to children with type 1 and type 2 diabetes.
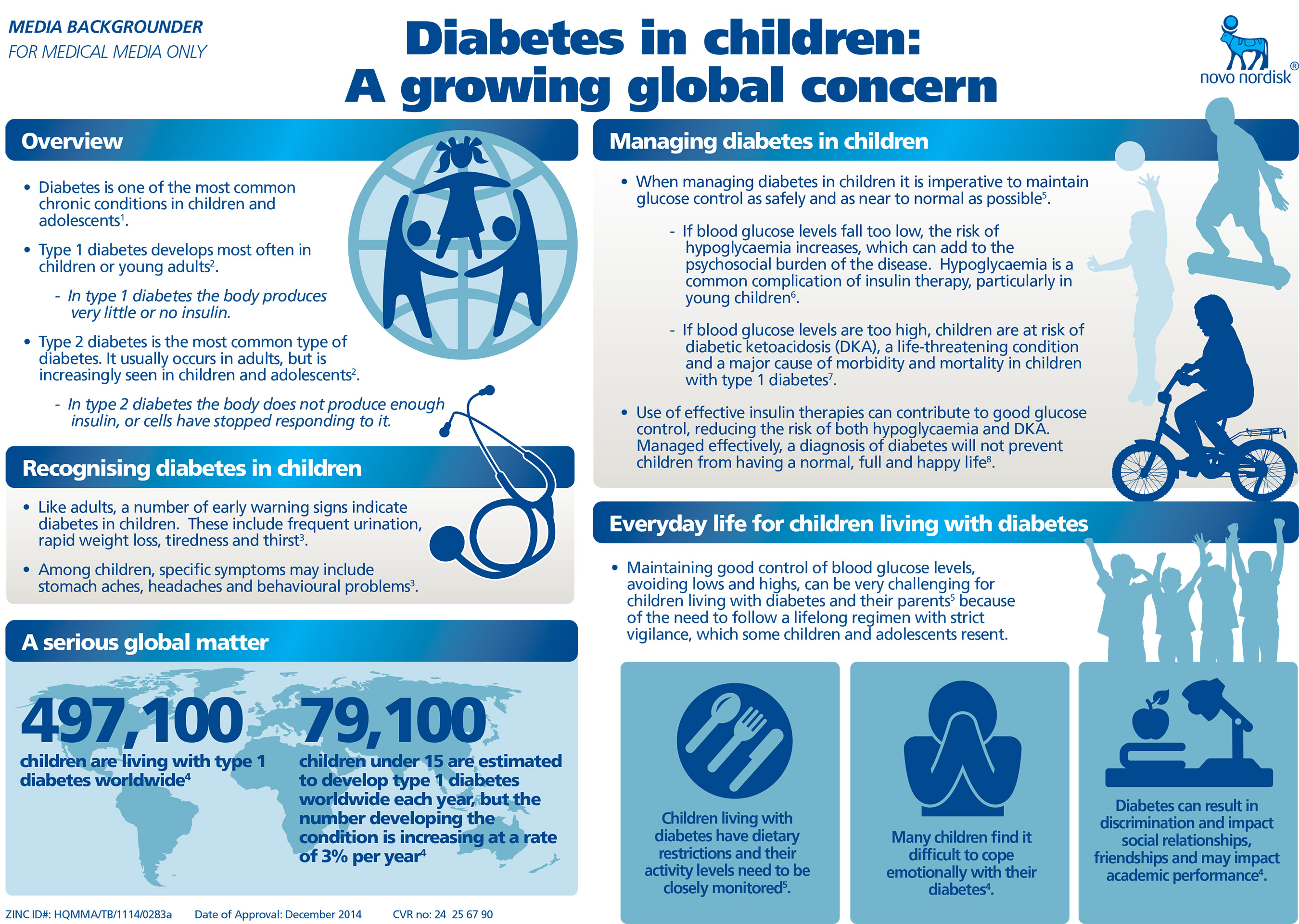
According to the International Diabetes Federation, an estimated 497,100 children are living globally with type 1 diabetes and rates of type 2 diabetes among children are also on the increase1.
"When treating children and adolescents with diabetes, getting patients to target while minimising side effects is always a priority,” said Dr Nandu Thalange, paediatric endocrinologist at Norfolk and Norwich University Hospital, Norwich, United Kingdom and the lead study investigator of the BEGIN® YOUNG 1 trial. “This latest CHMP recommendation for Tresiba® offers patients between the ages of 1 and 17 a new once-daily basal insulin which allows patients to get to target with a reduced risk of hyperglycemia with ketosis versus insulin detemir.”
The positive CHMP opinion for expanded use of Tresiba® in children and adolescents is based on efficacy and tolerability data from the BEGIN® YOUNG 1 trial, which is the first study to look into the long-term safety of Tresiba® in children with type 1 diabetes. Results show that Tresiba® given once daily in combination with insulin aspart effectively improved long-term glycaemic control2.
Tresiba® was approved in Europe in 2013 for once-daily use in adults with type 1 and type 2 diabetes as a monotherapy and in combination with oral anti-diabetic (OAD) medicinal products or with mealtime insulin. In May 2014, Tresiba® was approved for combination use with GLP-1 receptor agonists3.
About the study
The BEGIN® YOUNG 1 trial was a randomised controlled, 26-week open-label, treat-to-target trial (with a 26-week extension) investigating the efficacy and safety of Tresiba®, given once daily, and insulin detemir, given once or twice daily, both in combination with bolus insulin aspart in children and adolescents with type 1 diabetes2.
Tresiba® met the primary endpoint of non-inferiority to insulin detemir for mean change in HbA1c (p<0.05) at 26 weeks. In the 26-week extension a lower insulin dose and a significantly greater reduction in fasting plasma glucose (FPG*) versus insulin detemir (p<0.05) was achieved2. Both regimens had similar rates of overall and nocturnal hypoglycaemia, and the rate of severe hypoglycaemia was numerically higher with Tresiba® plus insulin aspart2. Of note, patients on Tresiba® had significantly lower rates of hyperglycaemia with ketosis (p<0.05)2. Weight (measured as SD score†) increased with Tresiba® and remained unchanged with insulin detemir2. Adverse event profiles were similar for Tresiba® and insulin detemir2.
About Tresiba®
Tresiba® (insulin degludec) is a once-daily basal insulin that provides an ultra-long duration of action beyond 42 hours3,4. It is important for people with type 1 and type 2 diabetes to establish a routine for insulin treatment. On occasions when administration at the same time of day is not possible, Tresiba® allows for flexibility in day-to-day dosing time when needed3,5,6.
Tresiba® has received regulatory approval in Argentina, Aruba, Azerbaijan, Bangladesh, Bosnia & Herzegovina, Brazil, Chile, Colombia, Costa Rica, El Salvador, the EU, Honduras, Hong Kong, Iceland, India, Israel, Japan, Kazakhstan, Lebanon, Lichtenstein, Macedonia, Mexico, Nepal, Norway, Russia, South Korea, Switzerland and the UAE.
About Novo Nordisk
Headquartered in Denmark, Novo Nordisk is a global healthcare company with more than 90 years of innovation and leadership in diabetes care. The company also has leading positions within haemophilia care, growth hormone therapy and hormone replacement therapy. Novo Nordisk employs approximately 40,700 employees in 75 countries, and markets its products in more than 180 countries. For more information, visit novonordisk.com.
Further information
|
Media: | ||
| Katrine Sperling | +45 4442 6718 | [email protected] |
|
Investors: | ||
| Kasper Roseeuw Poulsen | +45 3079 4303 | [email protected] |
| Daniel Bohsen | +45 3079 6376 | [email protected] |
| Melanie Raouzeos | +45 3075 3479 | [email protected] |
| Frank Daniel Mersebach (US) | +1 609 235 8567 | [email protected] |
References
- International Diabetes Federation. Diabetes Atlas, sixth edition. The global burden. Available at: https://www.idf.org/sites/default/files/EN_6E_Ch2_the_Global_Burden.pdf Last accessed: November 2014.
- Thalange N, et al. Long-term efficacy and safety of insulin degludec in combination with bolus insulin aspart in children and adolescents with type 1 diabetes. Diabetologia. 2014;57(Suppl.1): S395.
- Tresiba® Summary of Product Characteristics. Bagsværd, Denmark, Novo Nordisk A/S; 2014.
- Haahr H, Heise T. A review of the pharmacological properties of insulin degludec and their clinical relevance. Clin Pharmacokinet. 2014;53:787-800.
- Meneghini L, et al. The efficacy and safety of insulin degludec given in variable once-daily dosing intervals compared with insulin glargine and insulin degludec dosed at the same time daily. Diabetes Care. 2013;36:858-64.
- Mathieu C, et al. Efficacy and safety of insulin degludec in a flexible dosing regimen vs insulin glargine in patients with type 1 diabetes (BEGIN: Flex T1): a 26-week randomized, treat-to-target trial with a 26-week extension. J Clin Endocrinol Metab. 2013;98:1154-62.
* FPG measures the concentration of glucose in the plasma after the patient has not eaten for at least eight hours.
† Standard deviation (SD) measures the amount of variation from the average. A low standard deviation indicates that the data points are close to the average; a high standard deviation indicates that the data points are spread out over a large range of values.
ZINC ID#: HQMMA/TB/1114/0283