Emisphere Launches Eligen B12™, the First Oral Prescription Tablet Proven to Normalize B12 Levels Without the Need for an Injection
- Oral tablet offers prescription strength B12 option for the estimated 48 million Americans with a B12 deficiency
- Eligen® Technology being studied across multiple disease states, including diabetes
- Eligen B12 marks Emisphere’s expansion into commercializing practical innovations
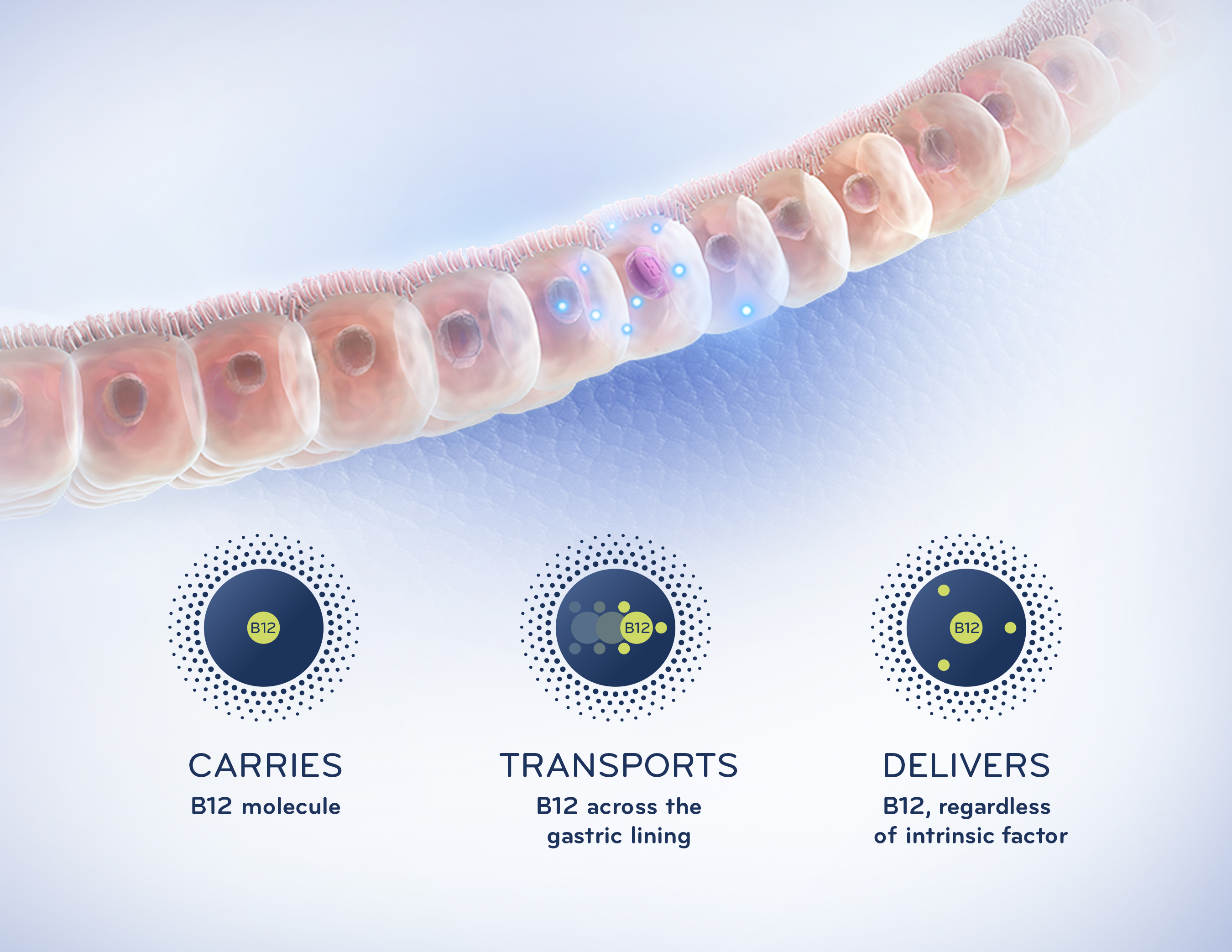
Eligen B12 is the first product to market using Emisphere’s advanced Eligen® Technology, which utilizes a carrier, salcaprozate sodium or SNAC, to chaperone B12 through the gastric lining and directly into the bloodstream independent of intrinsic factor, a protein made in the stomach that normally facilitates B12 absorption. This groundbreaking technology may have potential application across a broad range of diseases and conditions, including diabetes and womens health needs. Most notably, Eligen Technology is being used to develop oral formulations of insulin and GLP–1 receptor agonists.
“The launch of Eligen B12 is a transformational event, not only for Emisphere and its many supporters who have helped us achieve this milestone but, more importantly, for the patients who have been waiting for an effective oral alternative to treat vitamin B12 deficiency,” said Emisphere Chief Executive Officer Alan L. Rubino. “Eligen B12, the first of multiple products in development that use Eligen Technology, fills a significant gap in the B12 treatment landscape, offering prescription strength B12 that is clinically proven to normalize B12 levels without the need for an injection. This type of practical innovation is the backbone of our commercial expansion, one that can address a number of existing unmet medical needs while having a meaningful impact on the lives of patients.”
Medical B12 deficiency is a serious medical problem that, if left untreated, can cause irreversible damage to nerve cells and other co-morbidities. It can occur with a range of conditions in which there is an impaired capacity to absorb vitamin B12, including pernicious anemia, Crohn’s and celiac disease. Long term use of proton pump inhibitors (PPIs), a commonly used treatment to reduce acid in the stomach, also can impair the body’s ability to absorb B12.
“Most people – even those who are at highest risk like the elderly and folks with gastrointestinal problems – aren’t aware of the detrimental effects that a deficiency in vitamin B12 can have on their overall health,” Ralph Green, M.D., Ph.D., FRCPath, Medical Director, UC Davis Health System Medical Diagnostics Outreach Laboratory and a noted authority on vitamin B12, said during a recent interview regarding a new study published in 2015: Combined indicator of vitamin B12 status. “B12 deficiency is often overlooked, yet early detection and management is crucial because if untreated, it can lead to permanent nerve damage and serious neurological problems, such as memory loss and even dementia. It’s very important that anyone with a GI-related disorder and those over the age of 60, talk to their physician about being tested for B12 deficiency.”
The launch of Eligen B12 marks a turning point for Emisphere as it advances into a full realization of a well–calculated commercial expansion strategy. As the company’s first prescription product built on the groundbreaking Eligen Technology platform, Eligen B12’s commercialization has been precisely implemented through a fully integrated and comprehensive go–to–market strategy that includes a nationally deployed field force, a full–scale retail pharmacy roll–out, a highly targeted advertising and promotional campaign, and a sophisticated medical education component.
“Throughout the commercialization process, we placed unmet patient needs as a top priority and made product availability a key business imperative,” Rubino said. “As a result, B12–deficient patients across the country can have less concern about fluctuations in availability of other therapies for the management of B12 deficiency, because they now have reliable access to a new groundbreaking oral therapy with Eligen B12 which can achieve the same therapeutic goals.”
ABOUT ELIGEN B12
Eligen B12 is indicated for the dietary management of patients who have a medically–diagnosed vitamin B12 deficiency, associated with a disease or condition that cannot be managed by a modification of the normal diet alone. Eligen B12 is designed so that patients only need to take a single oral tablet (cyanocobalamin 1000 mcg/salcaprozate sodium [SNAC] 100 mg) of B12 daily.
Eligen B12 is the first and only medical food that has been shown to normalize vitamin B12 levels comparable to an intramuscular (IM) injection of B12. In a study that compared the impact of Eligen B12 and IM B12 on plasma B12 levels in 50 patients with demonstrated B12 deficiency (serum B12 <350 pg/mL), both products normalized B12 levels by Day 15 (first observation) and maintained normal levels over the duration of the study (three months). In a study that compared bioavailability in 20 healthy subjects of Eligen B12 with that of a standard oral B12 supplement, the bioavailability of Eligen B12 was 5.09 percent compared with 2.16 percent, which is more than double the bioavailability of the conventional over–the–counter oral B12 supplement formulation at the same dose.
Eligen B12 is classified by the U.S. Food and Drug Administration as a medical food. A medical food is a product formulated to be consumed or administered orally under medical supervision for the treatment of a disease or condition that cannot be managed by a modification of the normal diet alone.
ELIGEN B12 IMPORTANT SAFETY INFORMATION
Those with an allergy to B12, cobalt or any ingredients of Eligen B12 should not take this product. Eligen B12 should not be taken by people who have Leber’s disease, which physicians may refer to as hereditary optic nerve atrophy. Cyanocobalamin (B12) can lead to optic nerve damage (and possibly blindness) in people with Leber’s disease. Note that Eligen B12 has not been studied in patients below 18 years of age.
ABOUT EMISPHERE
Emisphere Technologies, Inc. is a specialty pharmaceutical company that has recently commenced commercial operations. Emisphere utilizes its proprietary Eligen Technology to create new oral formulations of therapeutic agents. Emisphere is currently partnered with global pharmaceutical companies for the development of new orally delivered therapeutics. For more information, please visit www.emisphere.com.
MEDIA CONTACTS:
Debbie Etchison
(212) 849-9450
[email protected]
Sara Baker
(212) 849-9474
[email protected]
INVESTOR CONTACTS:
Matthew Haines/Susan Kim
Argot Partners
(212) 600-1902
[email protected]
[email protected]
Photo Gallery
Related Links
Emisphere.comwww.KnowYourB12.com

Related Documents
B12 Deficiency InfographicFact Sheet