LINX System - Abridged Brief Statement
LINX® Reflux Management System - Abridged Brief Statement
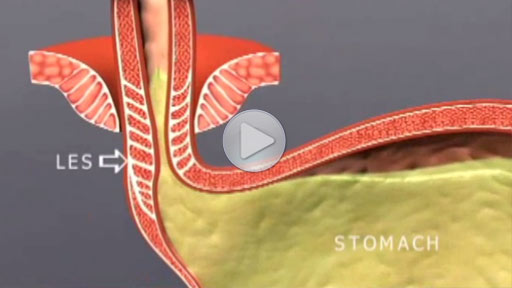
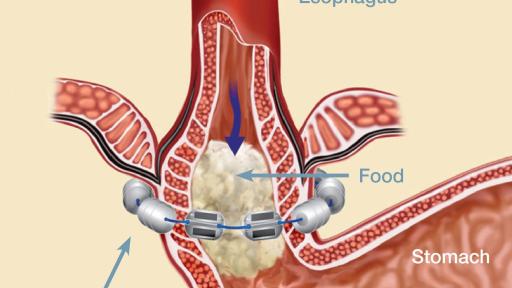
The LINX® Reflux Management System is indicated for those patients diagnosed with Gastroesophageal Reflux Disease (GERD) as defined by abnormal pH testing, and who continue to have chronic GERD symptoms despite maximum medical therapy for the treatment of reflux.
Caution: Federal law (USA) restricts these devices to sale by or on the order of a physician.
Contraindications: Do not implant the LINX System in patients with suspected or known allergies to titanium, stainless steel, nickel or ferrous materials.
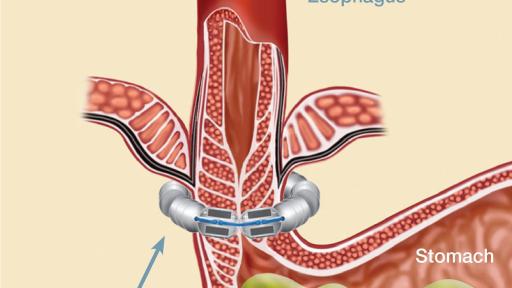
Warnings: The LINX device is considered MR Conditional in a magnetic resonance imaging (MRI) system up to either 0.7 Tesla (0.7T) or 1.5 Tesla (1.5T), depending on the LINX model implanted. Laparoscopic placement of the LINX device is major surgery.
General Precautions: The LINX device is a long-term implant for use in patients 21 years or older. Medical management of adverse reactions may include explantation and/or replacement.
Potential Risks Associated with LINX System: dysphagia, stomach bloating, nausea, odynophagia, increased belching, decreased appetite, inability to belch or vomit, flatulence, early satiety, device erosion, device migration, infection, pain, and worsening of preoperative symptoms.
For more information on the LINX Reflux Management System, contact your physician or Torax Medical, Inc.
For full patient information visit www.linxforlife.com or www.toraxmedical.com.