For further information please contact:
|
Donna Wright Ruder Finn [email protected] Tel: +44 (0)20 7438 3085 |
Mindy Dooa |
DIFICLIRTM▼(fidaxomicin) significantly reduces recurrence and all-cause mortality when used first-line in all patients with Clostridium difficile infection (CDI)
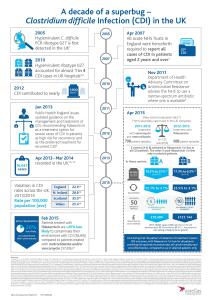
In the UK, there were 16,947 reported cases of CDI between April 2013 and March 20141,2,3,4 and in 2012 CDI contributed to nearly 1,900 deaths5,6,7
BLED, Slovenia, May 19, 2015 /PRNewswire/ - Data presented today from the CDI Service Evaluation study shows that the adoption pattern of treatment impacts CDI outcomes. Compared to traditional broad-spectrum antibiotics, first-line use of fidaxomicin - a targeted treatment - in all CDI patients provides improved outcomes in terms of recurrence rate, all-cause mortality and cost effectiveness, compared to use in selected patients only.8 CDI is associated with high-mortality5,6,7 and cost burden,9 therefore reducing the incidence and recurrence of CDI is a priority for clinicians, payers and health authorities alike.
Presented today at the 5th International Clostridium Difficile Symposium (ICDS) in Bled, Slovenia, the CDI Service Evaluation Study analysed a large dataset of over 1,450 CDI patient episodes. As a real-world multi-centre study, it assessed the effectiveness of current CDI treatment for UK patients in NHS Secondary Care Trusts in England.8
"CDI is a significant burden for patients and the NHS, and reducing its incidence and recurrence is a priority", comments Dr Simon Goldenberg, Consultant Microbiologist and Infection Control Doctor, Guy's and St Thomas' NHS Foundation Trust. "This study builds on the growing evidence that adopting fidaxomicin as first-line treatment for all patients with CDI, rather than reserving it for more severe cases, provides more optimal outcomes in terms of recurrence, all-cause mortality and cost effectiveness compared to older treatments - vancomycin and metronidazole. A previous study also showed that first-line use of fidaxomicin could reduce environmental contamination compared to those treated with vancomycin or metronidazole, further demonstrating the role fidaxomicin may play in reducing the spread and incidence of CDI alongside stringent hospital hygiene protocols."
Over 1,450 patient episodes were included in the analysis conducted in seven UK hospitals that introduced fidaxomicin, a narrow-spectrum antibiotic for the treatment of confirmed CDI in adults, between July 2012 and July 2013.8 Data collected were compared with a retrospective cohort treated with broad-spectrum antibiotics - vancomycin or metronidazole - during the previous 12-month period.8
Patterns of fidaxomicin use differed between the seven centres, with centres A and B treating with fidaxomicin as first-line treatment for all patients and the other five centres using fidaxomicin as first-line treatment only in selected patients for both primary and recurrent CDI.8
Data collected on 177 patient episodes treated first-line with fidaxomicin in centres A and B, showed a significant reduction in 28-day all-cause mortality, from 18.2% to 3.1% (P<0.001) and 17.3% to 6.3% (P<0.05) respectively.8,10 The real-world analysis also supports clinical trial data in highlighting dramatically reduced recurrence rates: from 12.1% and 23.5% in centres A and B with standard of care treatments (vancomycin and metronidazole), to 3.1% in both these centres where fidaxomicin was used first-line.8 For every 50 patients treated, this would result in 5 and 10 recurrences avoided in the two centres respectively.8
"Good antimicrobial stewardship includes using, where possible, narrow-spectrum rather than a broad-spectrum treatment," comments Professor Mark Wilcox, Professor of Medical Microbiology, Leeds Teaching Hospitals & University of Leeds. "Fidaxomicin has limited activity against the 'good bacteria' in the gut and so can be considered to be a targeted treatment option. Preservation of the gut microflora likely contributes to the lower rates of recurrence seen after fidaxomicin treatment of CDI compared with those associated with broader-spectrum antibiotics like vancomycin."
A CDI recurrence adds an additional £20,249 on top of the £13,146 spent to treat the initial infection due to prolonged hospital stay, ICU stay, high cost drugs and the surgery necessary to tackle it, as seen in the real-world evidence.11 An in-depth costing analysis at the two centres that adopted fidaxomicin as a first-line treatment revealed that in centre A the 5 recurrences that could be avoided for every 50 patients treated with the narrow-spectrum antibiotic would result in a cost saving of £19,490, and in centre B, for the 10 recurrences avoided, a cost saving of £121,144.8 With 16,947 cases of CDI reported in the UK between April 2013 and March 2014,1,2,3,4 the potential NHS cost saving for the treatment of this potentially fatal condition is likely to be far greater.
Guidance from Public Health England recommends fidaxomicin as an initial treatment option for severe cases of CDI in patients at high risk for recurrence; in addition, fidaxomicin is the preferred treatment for recurrent CDI.12
NOTES TO EDITORS
About the CDI Service Evaluation study8
The CDI Service Evaluation Project is the first and only real-world multi-centre study assessing the effectiveness of current CDI treatment for UK patients in NHS Secondary Care Trusts. This evaluation looked specifically at the cost-effectiveness of fidaxomicin in clinical practice versus standard of care treatments (vancomycin and metronidazole) in seven trial centres from across the UK:
- Leeds Teaching Hospitals NHS Trust
- Guy's and St Thomas' NHS Foundation Trust
- County Durham & Darlington NHS Foundation Trust
- University Hospitals of Morecambe Bay - NHS Foundation Trust
- St George's Healthcare NHS Trust
- University Hospitals of Leicester NHS Trust
- Derby Hospitals NHS Foundation Trust
Its full results were presented at the 5th International Clostridium Difficile Symposium (ICDS) in Bled, Slovenia (19-21 May 2015).
The study was sponsored by Astellas Pharma Ltd.
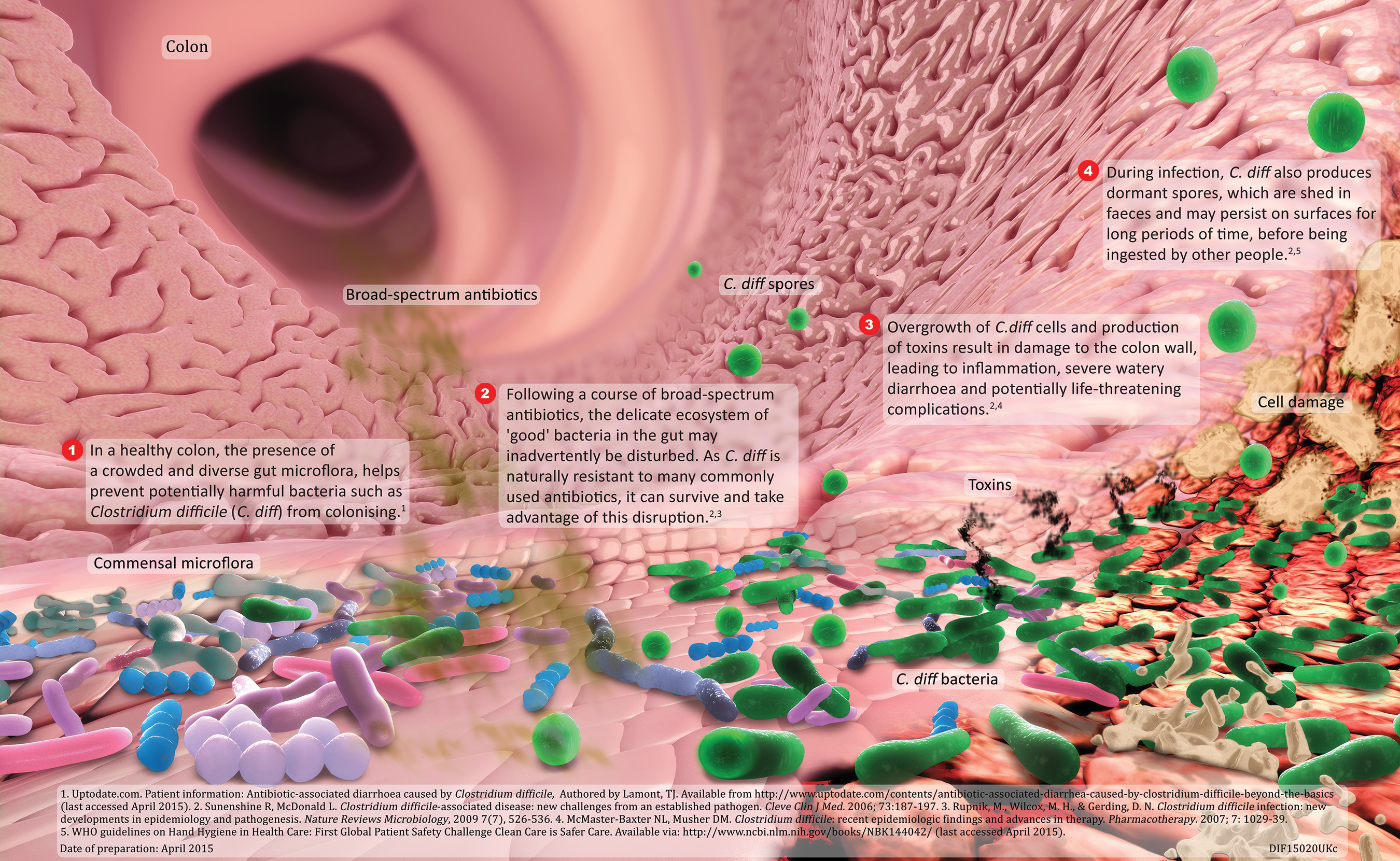
About Clostridium difficile Infection (CDI)
CDI is a potentially serious illness resulting from infection of the internal lining of the colon by Clostridium difficile bacteria. The bacteria produce toxins that cause inflammation of the colon, diarrhoea and, in some cases, death. Patients typically develop CDI after the use of broad-spectrum antibiotics that disrupt normal bowel flora, allowing C. difficile bacteria to flourish. The majority of CDI cases are reported in people aged over 65,13 but younger patients are also affected. CDI results in substantial costs to healthcare systems, in particular because of extended hospitalisation.14
About DIFICLIRTM (fidaxomicin)
DIFICLIR is a first-in-class macrocyclic antibiotic, of which the active substance is fidaxomicin.15 It is indicated for the treatment of (CDI) also known as C. difficile-associated diarrhoea (CDAD).16 It is the first licensed antibiotic treatment for CDI since the 1950s.17 Fidaxomicin is a targeted narrow spectrum antibiotic with highly selective activity against C. difficile bacteria rather than other gut flora.18
About Astellas Pharma EMEA
Astellas Pharma EMEA operates in 40 countries across Europe, the Middle East and Africa, and is the EMEA regional business of Tokyo-based Astellas Pharma Inc. Astellas is a pharmaceutical company dedicated to improving the health of people around the world through the provision of innovative and reliable pharmaceuticals. The organisation's focus is to deliver outstanding R&D and marketing to continue growing in the world pharmaceutical market. Astellas presence in Europe also includes an R&D site and three manufacturing plants. The company employs over 4,500 people across the EMEA region. In 2013 Astellas was awarded SCRIP Pharmaceutical Company of the Year in recognition of its commercial success and pipeline development.
References
-
Public Health England, Clostridium difficile infection: annual data. C. difficile infections: quarterly counts by acute trust and CCG and financial year counts and rates by acute trust and CCG, May 2014. Available online via: https://www.gov.uk/government/statistics/clostridium-difficile-infection-annual-data Last accessed, May 2015.
-
Health Protection Scotland, Quarterly report on the surveillance of Clostridium difficile infection in Scotland, January - March 2014. July 2014. Available online via: https://www.hps.scot.nhs.uk/haiic/sshaip/ publicationsdetail.aspx?id=50174 Last accessed, May 2015.
-
HSCNI Public Health Agency, Summary Tables for Clostridium difficile and MRSA (Meticillin Resistant Staphylococcus aureus) cases 2006/07 to 2014/15. Available online via: https://www.publichealth.hscni.net/sites/default/files/ End%20August%202014.pdf Last accessed, May 2015.
-
Public Health Wales. Clostridium difficile and Staphylococcus aureus Bacteraemia Monthly Update. Available online via: https://www.wales.nhs.uk/sites3/page.cfm?orgid=379&pid=67899 Last accessed, May 2015.
-
Office for National Statistics, Statistical bulletin: Deaths Involving Clostridium difficile, England and Wales, 2012, August 2013. Available online via: https://www.ons.gov.uk/ons/rel/subnational-health2/deaths-involving-clostridium-difficile/2012/stb-deaths-involving-clostridium-difficile-2012.html Last accessed, May 2015.
-
General Register Office for Scotland. Clostridium Difficile (C.diff) Deaths. Deaths for which Clostridium difficile was mentioned on the death certificate (either as the underlying cause of death or as a contributory factor), Scotland, 2000-2013. Available online via: https://www.nrscotland.gov.uk/files//statistics/c-diff-deaths/2013/c-diff-2013-tab6.pdf Last accessed, May 2015.
-
Northern Ireland Statistics & Research Agency. Healthcare-Associated Infection. Deaths Registered with Clostridium difficile Mentioned on the Death Certificate, 2003-2013. Available at: https://www.nisra.gov.uk/demography/default.asp29.htm Last accessed, May 2015.
-
Howard P, et al. The impact of the introduction of fidaxomicin on the management of Clostridium difficile infection in 7 NHS secondary care Trusts in England. Poster presented at ICDS 2015.
-
Department of Health, NHS Outcomes Framework: Impact Assessment (5014), December 2010. Available online via: https://www.gov.uk/government/uploads/system/uploads /attachment_data/file/213792/dh_122953.pdf Last accessed, May 2015.
-
Howard P, et al. The impact of the introduction of fidaxomicin on the management of Clostridium difficile infection in 7 NHS secondary care Trusts in England. Abstract presented at ICDS 2015.
-
St George's: Cost-effectiveness of fidaxomicin as first-line treatment for Clostridium difficile infection. Presentation, 2014. Astellas Pharma Ltd Data on File DIF14036UK(2).
-
Public Health England, Updated guidance on the management and treatment of Clostridium difficile infection, May 2013. Available online via: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/321891/ Clostridium_difficile_management_and_treatment.pdf Last accessed, May 2015.
-
NHS Choices, Clostridium difficile. Available online via: https://www.nhs.uk/conditions/Clostridium-difficile/Pages/Introduction.aspx Last accessed, May 2015.
-
Dubberke E, et al . Attributable Outcomes of Endemic Clostridium difficile-associated Disease in Nonsurgical Patients. Emerg Infect Dis. Jul 2008;14(7):1031-1038. Available online via: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2600322/ Last accessed, May 2015.
-
Mullane K. Gorbach S. Fidaxomicin: first-in-class macrocyclic antibiotic. Expert Rev. Anti Infect Ther. 2011;9(7):767-77.
-
Dificlir summary of product characteristics. Available online via: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002087/WC500119705.pdf Last accessed, May 2015.
-
Levine DP. Vancomycin: a history. Clinical Infectious Diseases. 2006;42:S5-12.
-
Tillotson GS and Tillotson J. Clostridium difficile - a moving target. F1000 Medicine Reports. 2011;3:6 (doi:10.3410/M3-6). Available online via: https://www.ncbi.nlm.nih.gov/pmc/articles/ PMC3096886/pdf/medrep-03-06.pdf. Last accessed, May 2015.
For further information please contact:
Donna Wright
Ruder Finn
[email protected]
Tel: +44(0)20-7438-3085
Mindy Dooa
Astellas Pharma EMEA
[email protected]
Tel: +44(0)7826-912-339
DIF15020UK Date of preparation: May 2015
DIF15020UKt Date of preparation: May 2015
Images
Backgrounders

The CDI Service Evaluation Project was funded and supported by Astellas Pharma Ltd.